
Less than 40 percent of people over 65 have gotten the booster shot that became available in the fall, according to the CDC, despite the administration’s six-week push to increase vaccination in that age group ahead of the holidays. Individuals over 65 are most at risk for serious illness from Covid, including hospitalization and death, and CDC data shows that 70 percent of average new daily hospital admissions of patients with confirmed Covid-19 over the last week were 60 and older. The announcement comes as the Biden administration has aggressively promoted the updated vaccine to older Americans.
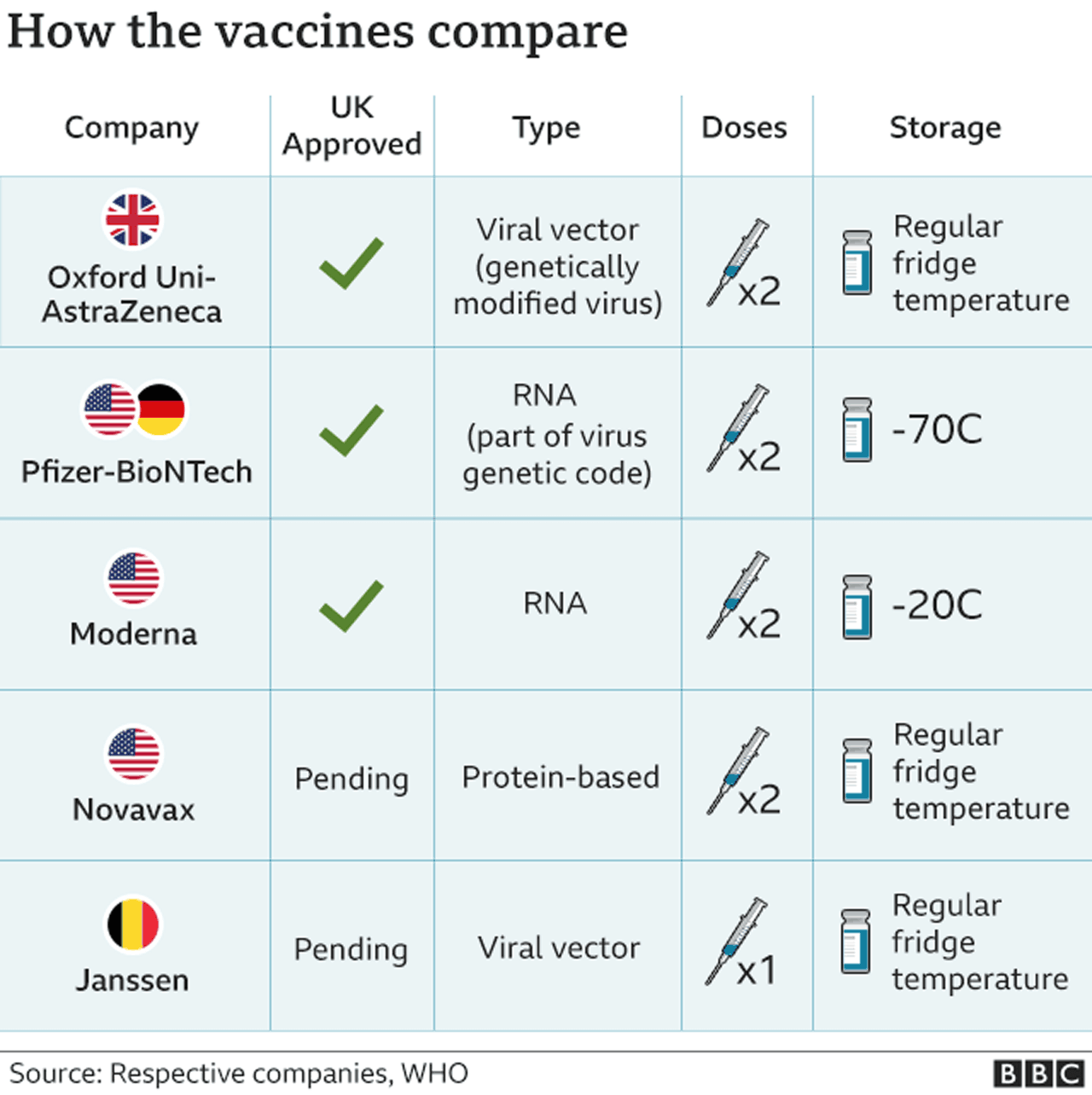
“If there’s one lesson that the CDC and FDA should have taken away from the pandemic, it’s the importance of providing honest, clear, precise, and timely information to the American people about the potential risks and benefits of COVID-19 interventions, including vaccination.” “These agencies must rapidly investigate, in an open and transparent manner, whether or not the vaccine may have contributed to the reported strokes,” McMorris Rodgers said in a statement. The announcement prompted a response from House Energy and Commerce Chair Cathy McMorris Rodgers (R-Wash.), who said CDC and FDA will testify before her committee. Pfizer and BioNTech said in a statement that there is “there is no evidence to conclude that ischemic stroke is associated with the use of the companies’ COVID-19 vaccines.” The real-time surveillance system, CDC’s Vaccine Safety Datalink, met criteria warranting further investigation into whether the bivalent Pfizer vaccine led to a higher risk of ischemic stroke, which occurs when arteries pumping blood to the brain are blocked by a blood clot. Nearly 15% of vaccine participants got a fever after either the first or second dose, according to the FDA.“Although the totality of the data currently suggests that it is very unlikely that the signal … represents a true clinical risk, we believe it is important to share this information with the public, as we have in the past, when one of our safety monitoring systems detects a signal,” the statement said. Like Pfizer's Covid vaccine, which the FDA authorized last week, Moderna's vaccine similarly requires two shots separated by a few weeks. The FDA noted that more severe "serious adverse reactions occurred in 0.2% to 9.7% of participants" and were more common after the second dose than the first. More than 44% of people who received the vaccine reported experiencing joint pain and over 43% reported chills.
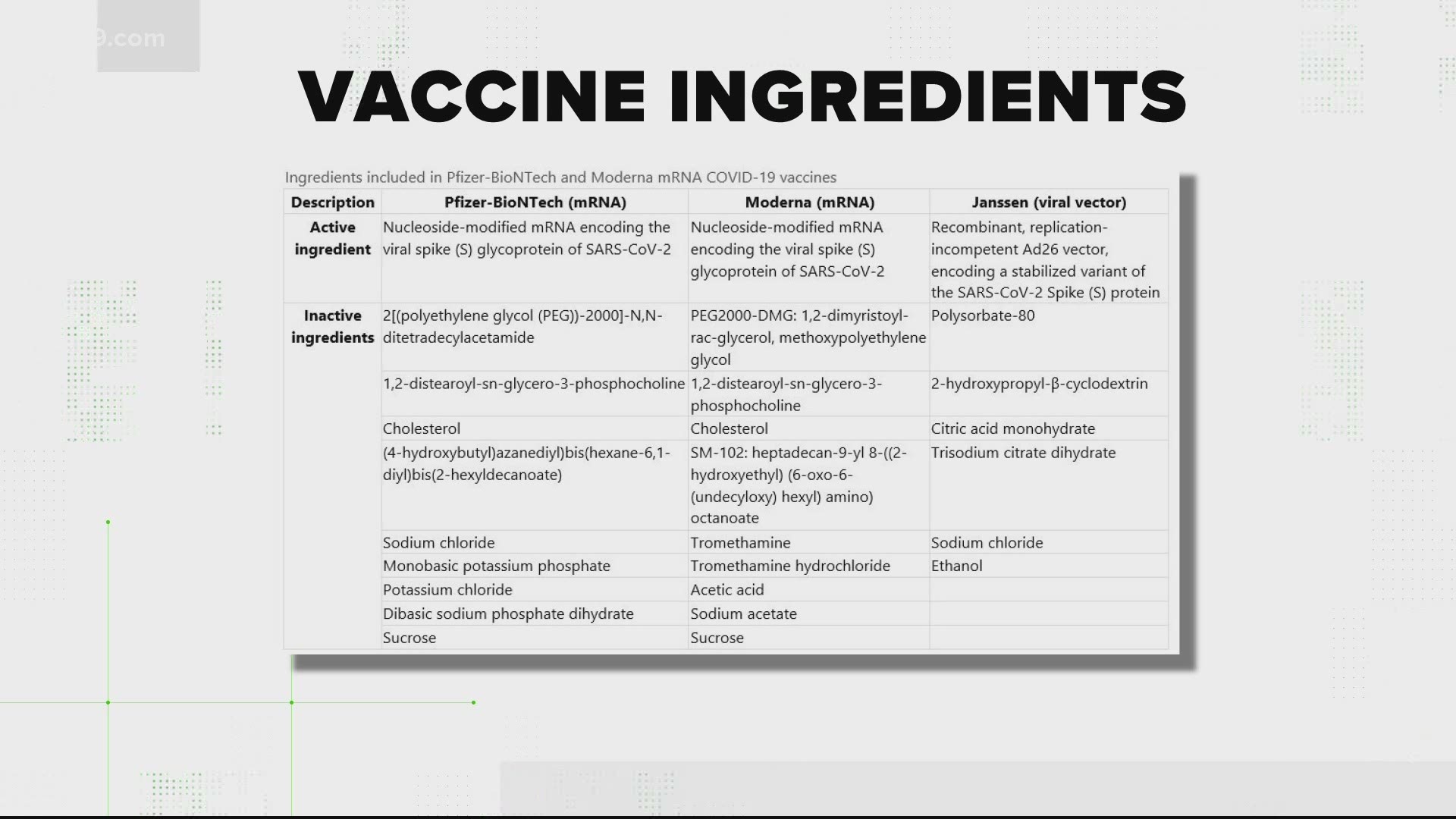
More than 9 in 10 participants who received the vaccine felt pain at the injection site, almost 7 in 10 felt fatigued and roughly 6 out of 10 had headaches or muscle pain, the FDA said. But the regulatory agency's analysis noted that the vaccine is associated with common and unpleasant, but not necessarily dangerous side effects. Moderna's vaccine, which was endorsed by FDA staff Tuesday, is more than 94% effective and safe enough to meet agency's bar for emergency use, according to the report. Many physicians are advising the public to brace for some stronger-than-usual side effects from the Covid-19 shots than, say, a typical flu shot, and to possibly take a day or two off work to recover. It's actually an immune response that indicates the shots are working as intended, doctors say. Personal Loans for 670 Credit Score or Lower

Personal Loans for 580 Credit Score or Lower Best Debt Consolidation Loans for Bad Credit


 0 kommentar(er)
0 kommentar(er)
